Yellow Card - MHRA
com.mhra.yellowcard
Total installs
100+
Rating
5.0(1 reviews)
Released
July 3, 2015
Last updated
March 1, 2023
Category
Medical
Developer
MHRA
Developer details
Name
MHRA
E-mail
unknown
Website
unknown
Country
unknown
Address
unknown
Screenshots

Description
The Yellow Card Scheme is the UK system run by the MHRA* for collecting and monitoring information on suspected adverse reactions to all medicines including vaccines, blood factors and immunoglobulins, herbal medicines and homeopathic remedies, and all medical devices available on the UK market.
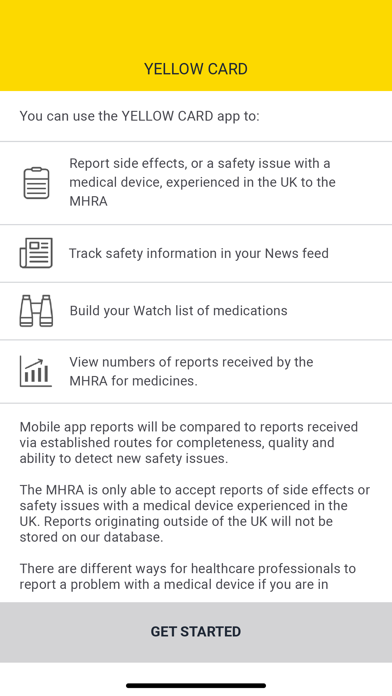
The Yellow Card app has been developed for medicines and allows users to:
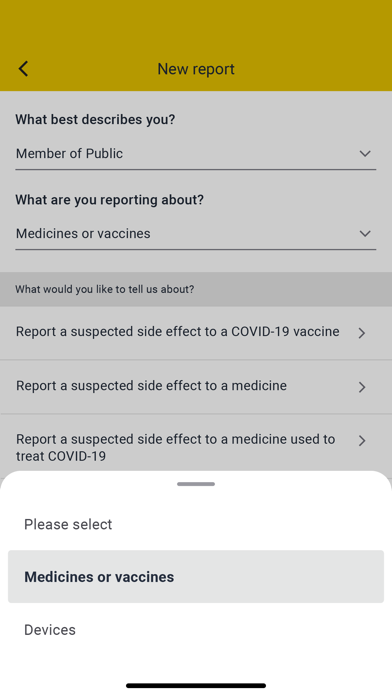
> Report a suspected side effect to a medicine (including vaccines, herbal products and homeopathic remedies)
> Track new safety information published by the MHRA about medicines
> Create a watchlist for alerts to medicines of interest to you
> View numbers of reports received by the MHRA to medicines and vaccines
Suspected problems or incidents involving medical devices, defective medicines, suspected counterfeits or fake medicines can also be reported to the MHRA via the Yellow Card website at http://www.mhra.gov.uk/yellowcard. All reports received through the app or website can help the MHRA identify potential new concerns about a medicine or medical device and act as an early warning for further investigation. The MHRA will review the product if necessary, and take action to minimise risk and maximise benefit to the patients.
*The Medicines and Healthcare products Regulatory Agency (MHRA) is the executive Agency of the Department of Health and Social Care; the MHRA protects and promotes public health and patient safety by ensuring that medicines, healthcare products and medical equipment are used safely and meet appropriate standards of safety, quality, performance and effectiveness.